SERVICES
2M is a strategic partner for start-ups and new medical technologies in the Life Science Industry, in national and international markets. 2M can support in several stages of the life science chain, which begins with a research innovation in one of the life sciences fields and leads to the launch of a fully developed product.
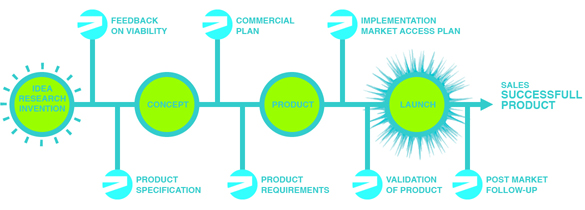
Feedback on viability
Once the research invention or idea has been born 2M Life Sciences can help answer the first important commercially related questions before the proof of concept experiments are designed. Is this a viable product or proposition? The answers to these important questions make sure that the unnecessary investments are not made for a product that is not commercially viable and that the proof of concept results meet the expectations of the proposed customer. To answer the most important question: Is it a sellable product or proposition?
- Who are the potential customers for the product/proposition?
- Which need does this product /proposition fulfil?
- How does this impact clinical management?
- Can the new technology replace other techniques?
- What is the (clinical)value of this invention? Define the clinical pathway.
- Is there enough evidence conduct an early economic analysis?
- Who else will interact with this product than the primary customer? (Stakeholders)
- Does the product/proposition fit the needs of the different stakeholders
- What is the business model?
- Is it potentially scalable?
- What are the clear advantages versus competitors?
Other important questions to be answered once a sellable product has been defined:
- Should the invention be patented?
- What are the hurdles that need to be taken before launch? (e.g. quality, safety, efficacy, regulatory, reimbursement)
- What are the fields of expertise essential to the success of the product?
- How will we access this expertise’s? Hire partnerships or other and is this doable
Based on the answers the next steps can be taken to look for financing for the proof of concept experiments for example: initial business model en plan. If needed 2M Life Sciences can aid in developing these documents and connecting the inventor with potential financing options. Once this is secured the critical specifications for the product or proposition can be defined (see PRODUCT SPECIFICATION) ..
PRODUCT SPECIFICATION
Using the process of the clinical pathway, define the critical specifications that the product needs to meet. (e.g. sensitivity, specificity, time restraints, resource requirements)
- What are the absolute MUST for the these specifications?
- What are the acceptable levels for these specifications?
COMMERCIALIZATION PLAN
Now that the technology has been proven to work, the next step can be taken to start a venture. 2M Life Science can then help to further refine the business model for the next financing round and in addition make sure that the to be designed product is defined appropriately: If appropriate 2M Life Sciences can also link to other expertise’s in their vast network.
Essential elements of this plan are:
Market What are existing products on that market? What are the products used for? Who buys and uses the product? What is the market size? What are other key characteristics of the market, is the product a commodity, or is the market differentiated in different products? Is the market regional or international and, if so, what are relevant differences in market sizes and conditions in different countries?
Product
What is your product? How does it compare to existing products? What is the target group?
Competitors
What are the competitors? What are their strengths and weaknesses? What are their current market shares? How did they achieve this market share? What are their strategies and ambitions?
Substitutes
Can the product be substituted by other products? If so, assess the impact of this possibility (i.e. how does the quality, price etc. of the substitutes compare with that of your product?)
Entry hurdles
How easy/difficult is it for other/new companies to enter your market and how does that impact the marketability of your product? Are these hurdles doable?
Sales distribution channel
Who are the suppliers? What is your relationship with these suppliers? If no relationship how can a relationship be built?
Customers/Buyers/Stakeholders
Assess your relationship with your buyers. How strong is your negotiating position and how does your relationship with your buyers impact your ability to market the product?
Other Stakeholders
Other stakeholders have been defined in FEEDBACK ON VIABILITY. Asses your relationship with these stakeholders? How will these relationship be built or maintained?
Other Hurdles for Market Access
Regulatory? Reimbursement? Customer Acceptance Define how these will be addressed.
Marketing Mix
What is the proposed marketing mix with which you will market the product? Pricing What is the intended sales price of the product and how does this price compare to other products: what is the justification of the chosen sales price?
Marketing Communication Plan
Which message will be required for the different stakeholder that have been defined.
Sales & Distribution
How will you organize the sales of your product? Will you have distributors or market and distribute the product yourselves? What effort is required for this?
Service
What level of service is needed for the product? Who will you provide what level of service?
Expected Future developments
What relevant developments are expected? Are there are any meaningful trends that affect the commercial opportunities of your product? Do you foresee relevant regulatory changes?
Expected Future technical developments
What technical developments are expected, both in the company’s own product and in competing/substituting products? How will these developments impact the product’s market and competitive advantage?
Financials
What is the expected profitability of your product? What are the expected costs for product development, production and marketing and sales and what are the expected revenues that the product will generate?
Planning
What needs to be done when to market the product?
PRODUCT REQUIREMENTS
Before developing the product the product specifications need to be carefully evaluated and further defined.
- What are the customer and other stakeholders needs and wishes? (see FEEDBACK ON VIABILITY)
- How do these translate into product requirements?
- How do these translate into technical specifications? These answers are input for product design.
IMPLEMENTATION OF MARKET ACCES PLAN
Introduction In this phase 2M can...
- Build close relationships with important stakeholders including of course customer, Key Opinion leade
- Develop required partnerships
- Set up of marketing, sales and customer services departments
- Implement marketing communication plan
VALIDATION OF PRODUCT
In this phase the product will be clinically validated in field trials alongside these clinical validation the benefits should be quantified for marketing and commercial purposes.
- Collect feedback from customers and if applicable patients
- Translate benefits into marketing tools
- Feedback requiring attention requires follow-up so that this does not damage the customer satisfaction.
POST MARKET FOLLOW UP
The product now enters a succession of strategies as the product goes through the life cycle, each phase requiring different strategies.
- Extend to other markets internationally.
- Collect valuable feedback on the performance of the product
- Develop new marketing tools to further promote this
- Define updates and modifications for next generations of the product.