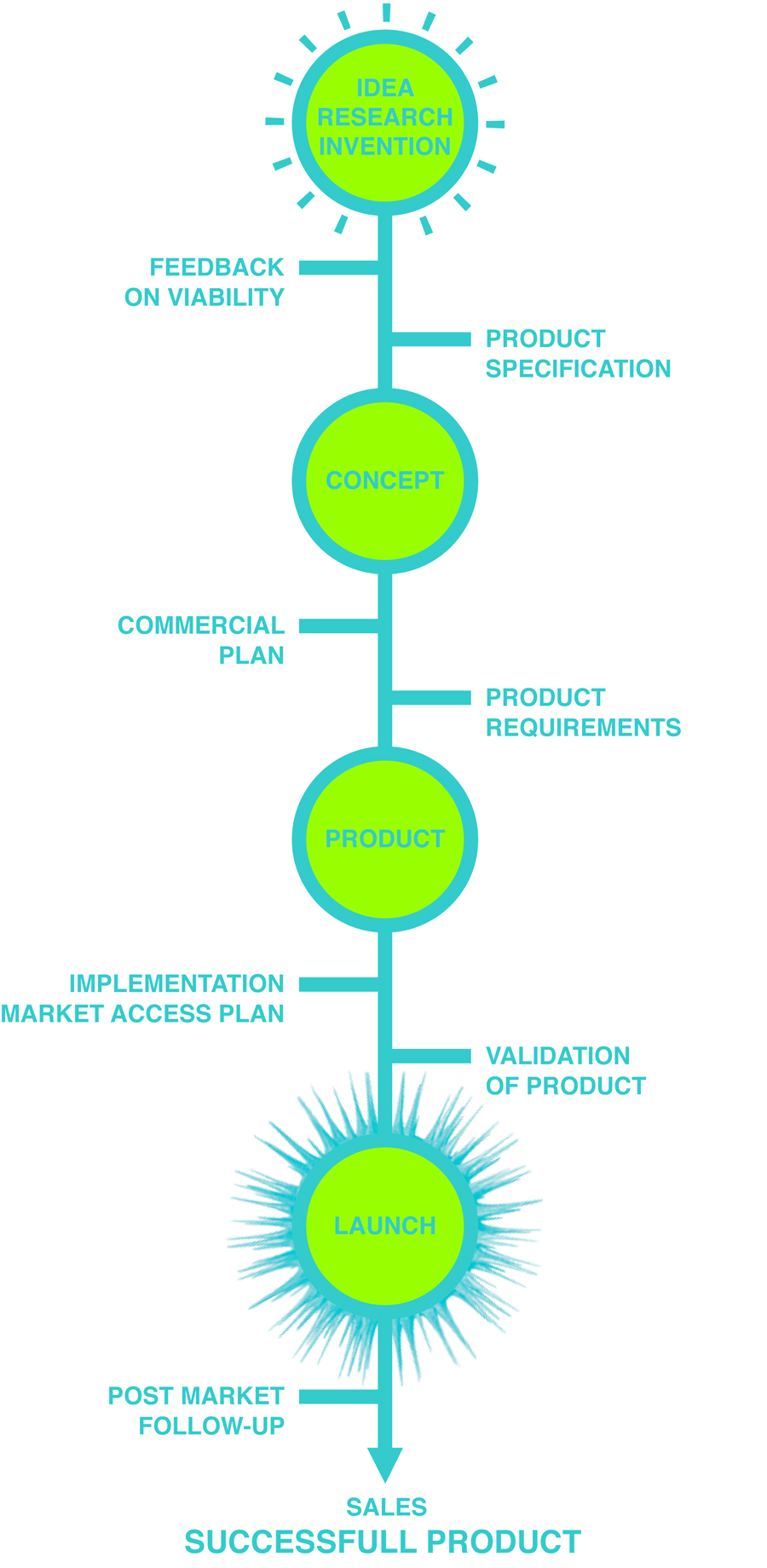
2M SERVICES & LIFE SCIENCE CHAIN
2M is a strategic partner for start-ups and new medical technologies in the Life Science Industry, in national and international markets. 2M can support in several stages of the life science chain, which begins with a research innovation in one of the life sciences fields and leads to the launch of a fully developed product.
ON VIABILITY COMMERCIAL
PLAN IMPLEMENTATION
MARKET ACCESS PLAN PRODUCT
SPECIFICATION PRODUCT
REQUIREMENTS VALIDATION
OF PRODUCT POST MARKET
FOLLOW-UP
COMMERCIAL PLAN
Now that the technology has been proven to work, the next step can be taken to start a venture. 2M Life Science can then help to further refine the business model for the next financing round and in addition make sure that the to be designed product is defined appropriately: If appropriate 2M Life Sciences can also link to other expertise’s in their vast network. Essential elements of this plan are:
More >>
Case >>
IMPLEMENTATION MARKET ACCESS PLAN
In this phase 2M can...
> Build close relationships with important stakeholders including of course customer, Key Opinion leade,
> Develop required partnerships
> Set up of marketing, sales and customer services departments
> Implement marketing communication plan
Case >>
PRODUCT SPECIFICATION
Using the process of the clinical pathway, define the critical specifications that the product needs to meet. (eg sensitivity, specificity, time restraints, resource requirements)
> What are the absolute MUST for the these specifications?
> What are the acceptable levels for these specifications?
Case >>
PRODUCT REQUIREMENTS
Before developing the product the product specifications need to be carefully evaluated and further defined.
> What are the customer and other stakeholders needs and wishes? (see FEEDBACK ON VIABILITY)
> How do these translate into product requirements?
> How do these translate into technical specifications?
These answers are input for product design.
Case >>
VALIDATION OF PRODUCT
In this phase 2M can...
> Build close relationships with important stakeholders including of course customer, Key Opinion leade,
> Develop required partnerships
> Set up of marketing, sales and customer services departments
> Implement marketing communication plan
Case >>
POST MARKET FOLLOW-UP
The product has now entered a succession of strategies as the product goes through the life cycle, each phase requiring different strategies.
> Extend to other markets internationally.
> Collect valuable feedback on the performance of the product
> Develop new marketing tools to further promote this
> Define updates and modifications for next generations of the product.
Case >>
Healthmark launched in the Corona drive thru
April 7th 2020 Corona drive thru set up in Leiden with the support of Healthmark software
Ieders eigen ideologische zuurstoffles
NRC, Robbert Dijkgraaf, 30 september 2016
"Waarom lukt het zo slecht de waarden van de wetenschap te delen? Waarden die tot de kern van iedere gezonde samenleving zouden moeten horen, zoals het zoeken naar waarheid, de wens tot nuance, een gezonde dosis scepsis, respect voor feiten én voor onzekerheden en verwondering over de rijkdom van de natuur en de menselijk geest." >